Krishna Surendar Karuppasamy

,
S. Ramesh Kumar

,
S. Krishnakumar

,
V. Varshini

,
N. Susithra

,
S. Kavitha

,
V. Rajendran

Vanavarayar Institute of Agriculture, VIA, Pollachi-642 103, India

Author

Correspondence author
Legume Genomics and Genetics, 2014, Vol. 5, No. 5 doi: 10.5376/lgg.2014.05.0005
Received: 24 Jun., 2014 Accepted: 16 Jul., 2014 Published: 22 Jul., 2014
This is an open access article published under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The experiment aimed at assessing the effects of the progressive salinity stress, as well as investigating the physiological behavior viz., soluble protein content, NRase enzyme activity, proline and catalase enzyme activity in green gram subjected to sodium chloride during the seed germination under laboratory conditions in the Vanavarayar Institute of Agriculture (TNAU affiliated), Pollachi, Tamil Nadu, INDIA. The parameters that were measured are the total soluble proteins, NRase enzyme activity, proline and catalase enzyme activity. The experimental design carried out was CRD, with eleven treatments and two cultivars of CO6 and CO8. There was decrease in the leaf relative water content and soluble protein content in plants under salinity, however NRase enzyme activity were increased by 32 per cent respectively. Indicating that the carbon metabolism is quickly modified and utilized as reserve source and membrane protector during the salinity stress. Besides this, there was an increase in proline and catalase enzyme activity was noticed to the salinity stress.
Salt stress; Seed germination; Soluble protein; NRase; Proline; Catalase; Green gram
1 Introduction
Green gram is the richest protein source of human diet and livestock in poor areas. Apart from that, they are used as green manures and green fodder to animals. Mainly they are used for fixing atmospheric nitrogen to improve the physical and chemical properties of soil. Among the legumes, Green gram was considered as the most important traditional crops of India. Salinity-an abiotic stress is an ever increasing problem that seriously affects crop production in various parts of the world, especially in areas where are irrigated with water containing salts. Salt stress is one of major factors in constraining crop adjustment substances, soluble sugar content, proline production.
About 23% of the world’s cultivated lands are saline and 37% is sodic (
Khan and Duke, 2001). Salinity affects 7% of the world’s land area of about 930 million hectares. Salinity reduces the yield of pulses by more than 50% (
Bray, 2000). Soils can be saline due to geo-historical processes or they can be man-made. The water and salt balance, just like in oceans and seas determine the formation of salty soils, where more salt comes in than goes out. Here, the incoming water from the land brings salts that remain because there is no outlet and the evaporation water does not contain salts. Soil salinity in agriculture soils refers to the presence of high concentration of soluble salts in the soil moisture of the root zone. Salt stress induces the synthesis of abscisic acid which closes stomata when transported to guard cells. As a result of stomatal closure, photosynthesis declines and photo inhibition and oxidative stress occur. Chlorophyll is the principal agent responsible for photosynthesis and, under adverse conditions, chlorophyll level is a good indicator of photosynthetic activity (
XinWen et al., 2008). The deleterious effect of salinity is increased osmotic pressure which restricts the absorption of water into the seeds (
Tester and Davenport, 2003). It is also toxic to the embryo and seedlings. Enzyme called α – amylase which is essential for seed germination is inhibited due to salt stress. Starch to sugar conversion occurs during germination is also affected by salinity. It also delays the synthesis of nucleic acids and RNAase. As regard to the chlorophyll content of the salinized plant, it is apparent that the chlorophyll content was reduced with increasing salinity. When salinity has affected the warning signs were sick or dying trees and declining vegetation. As salinity impacts on any remaining native vegetation and the wildlife that depends on it for survival, the loss of biodiversity escalates. Salinity also reduces the productivity of crops and the sustainability of agriculture. Based on the above constraints, we are taken the objective of Screening of Green gram varieties for NaCl stress tolerance through physiological analysis.
2 Materials and Methods
The experiment was carried out at Vanavarayar Institute of Agriculture (TNAU affiliated), Pollachi, Tamil Nadu, India during 2013-2014. The experiment consists of ten treatments with three replications were laid out in completely randomized block design with two cultivars of CO5 and CO6. Seeds of green gram varieties obtained from Department of Pulses, Tamil Nadu Agricultural University, Coimbatore, were used for the study and the details of the varietal characters were listed in
Table 1. Green gram varieties (
Table1) were screened for tolerance to various levels of sodicity stress, based on germination per cent, seedling growth and vigour index, seeds were allowed to germinate in Petri dishes. The germination medium was prepared following the procedure mentioned below. Petri dishes were sterilized using 0.01% HgCl
2 and 70% ethanol and finally washed with distilled water. Before placing the germination sheet, Petri dishes were cleaned thoroughly with a cotton swab. The surface sterilized (70% ethanol) 15 seeds from each variety were placed in each Petri dish. For imposing sodicity (11 levels as considered as Treatments) stresses, sodium chloride (NaCl) solution at the concentration of T
1: control (without NaCl), T
2:10, T
3:20, T
4:30, T
5:40, T
6:50, T
7:60, T
8:70, T
9:80, T
10:90 and T
11:100 ppm were prepared. The seeds were allowed to germinate, by sprinkling the salt solution of 10 mL each in alternate days. Distilled water was used for maintaining the control. The pH and EC details of the salt solution used for experiment were given in
Table 2.
.gif)
Table 2 pH and EC of the salt solution used for experiment
|
2.1 Observation recorded
Soluble protein content: Soluble protein content of leaf was estimated as per the method of
Lowry et al. (1951) and expressed as mg/g fresh weight.
2.2 Nitrate Reductase enzyme activity
Proline content of the leaf sample was estimated by the method of
Nicholas et al. (1976) and expressed as µg of NO
2 g
-1·hr
-1 of fresh weight.
2.3 Proline (mg/g)
Proline content of the leaf sample was estimated by the method of
Bates et al. (1973) and expressed as µg/g of fresh weight.
2.4 Catalase (mg/g)
Catalase activity of the leaf sample was assayed as per the procedure adopted by
Gopalachari (1963) and expressed as µg H
2O
2 g
-1·min
-1.
3 Result and Discussion
3.1 Soluble protein (mg/g)
The soluble protein content of the leaf, being a measure of RuBP carboxylase activity was considered as an index for photosynthetic efficiency. There were reports that RuBP-case enzyme forms nearly 80 per cent of the soluble proteins in leaves of many plants (
Joseph et al., 1981). The result on soluble protein content was significantly differed in all the treatments. Among the treatments, T1 showed highest Soluble protein content in green gram both CO6 and CO8 (4.19 and 9.16), which was followed by T
3, T
4 and T
5. The lowest soluble protein content was recorded in T
7, T
8 and T
11 treatments (
Table 3).
Martignone et al. (1987) observed that in soybean soluble protein content was the first nitrogenous compound affected under stress conditions, which at severity got denatured and lost the activity. It was further explained that soluble protein, world’s most abundant protein containing the enzyme RUBISCO, is involved in CO
2 assimilation; therefore, the reduction in soluble protein might have a direct adverse effect on photosynthesis.
3.2 Nitrate Reductase enzyme activity
The result on nitrate reductase activity was significantly differed in all the treatments. Among the treatments, T
2 showed highest nitrate reductase activity in green gram both CO6 and CO8 (83.60 and 85.15), which was followed by T
3, and T
4. The lowest nitrate reductase activity was recorded in T
10 and T
11 treatments (
Table 3).
.gif)
Table 3 Effect of salt stress (NaCl) on soluble protein and nitrate reductase enzyme activity of green gram (CO6 and CO8)
|
3.3 Proline (mg/g)
The result on proline content was significantly differed in all the treatments. Among the treatments, T2 showed highest proline content in green gram both CO6 and CO8 (382.71 and 384.70), which was followed by T3, T4, T5 and T6. The lowest proline content was recorded in T9, T10 and T11 treatments (Table 4). Rosa - Ibarra and Maiti (1995) under salinity stress reported that increase in proline content is probably due to the capacity of some plants to accumulate organic (sucrose, fructose and glucose) and inorganic (Na, K and Cl) metabolites in the cytoplasm to reduce the water potential and change the osmotic gradient, assuring the water flow to the plant and thereby increase tolerance.

Table 4 Effect of salt stress (NaCl) on proline and catalase enzyme activity of green gram (CO6 and CO8)
|
3.4 Catalase (mg/g)
The result on catalase activity was significantly differed in all the treatments. Among the treatments, T
2 showed highest catalase activity in green gram both CO6 and CO8 (77.10 and 72.02), which was followed by T
3, T
4 and T
5. The lowest catalase activity was recorded in T
8, T
9, T
10 and T
11 treatments (
Table 4). In this present study, we noticed that, the increase in enzyme activity with external salinity may be due to increased synthesis of enzyme. Transgenic plants over expressing ROS scavenging enzymes, such as super oxide dismutase (
Alscher et al., 2002), ascorbate peroxidase (
Wang et al., 1999) and glutathione S-transferase / glutathione peroxidase (
Roxas et al., 1997 and 2000) showed increased tolerance to osmotic and oxidative stresses.
3.5 Correlation studies
In this experiment result, the effect of sodium chloride on greengram variety CO6 and CO8 had positively correlated in proline and catalase enzyme activity. The variety CO6 had R
2 value of 0.9316 (
Figure 1) and CO8 had R
2 value of 0.9412 (
Figure 2).
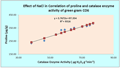
Figure 1 Correlation studies of proline and catalase enzyme activity in greengram CO6
|
.gif)
Figure 2 Correlation studies of proline and catalase enzyme activity in greengram CO8
|
Abdual-baki A.A., and J.D. Anderson, 1973, Relationship between decarboxylation of glutamic acid and vigour in soybean seed, Crop Sci., 13: 222-226
Blum A., Mayer J. and Andgolan G., 1989, Agronomical and Physiological assessments of genotypic variation for drought resistance in sorghum, Australian Journal of Agricultural Research, 16(1): 49-61
http://dx.doi.org/10.1071/AR9890049
Bray E.A., J. Bailey-Serres and E. Weretilnyk, 2000, Responses to abiotic stress, In: Buchanan B., Gruissem W. and Jones, R. (Eds.). Biochemistry and Molecular Biology of Plants. American Society of Plant Physiology. , Rockville, pp1158-1203.
Gopalachari N.C., 1963, Changes in the activities of certain oxidizing enzymes during germination and seedling development of Phaseolus mungo and Sorghum vulgare, Indian J. Exp. Biol., 1: 98-100
Hanson, 1978, Application of the chemiosmotic hypothesis to ion transport across the root, Plant Physiol., 42: 294-298
Joseph M.C., D.D. Randall and C.J. Nelson, 1981, Photosynthesis in polyploid tall fescue. II. Photosynthesis and RUBP case of polyploid tall fescue, Plant Physiol., 68: 894-898
http://dx.doi.org/10.1104/pp.68.4.894
Lapina I.P. and Popov, 1970, Effect of sodium chloride on the photosynthetic apparatus of tomatoes, Fiziol. Rast., 17: 580-585
Lowry O.H., Rosebrough N.J., Farr A.L., and Randall R.J., 1951, Protein measurement with the folin phenol reagent, J Biol Chem., 193: 265-275
Nicholas J.C., Harper J.E., and Hageman R.H., 1976, Nitrate Reductase activity in soybeans (Glycine max [L] Merr.): 1. Effects of light and temperature, Plant Physiology, 58: 731-735
http://dx.doi.org/10.1104/pp.58.6.731
Shao H.B., L.Y. Chu, Z.H. Lu, and C.M. Kang, 2008, Main antioxidants and redox signaling in higher plant cells, Int. J. Biol. Sci., 44: 12-18
Xinwen X., Hailiang X., Yangling W., Xiaojing W.,Yongzhi Q. and Bo X., 2008, The effect of salt stress on the chlorophyll level of the main sand – binding plants in the shelterbelt along the Tarim Desert Highway, Chinese Science Bulletin, 53: 109-111
http://dx.doi.org/10.1007/s11434-008-6012-5

 Author
Author  Correspondence author
Correspondence author
.gif)

.gif)


